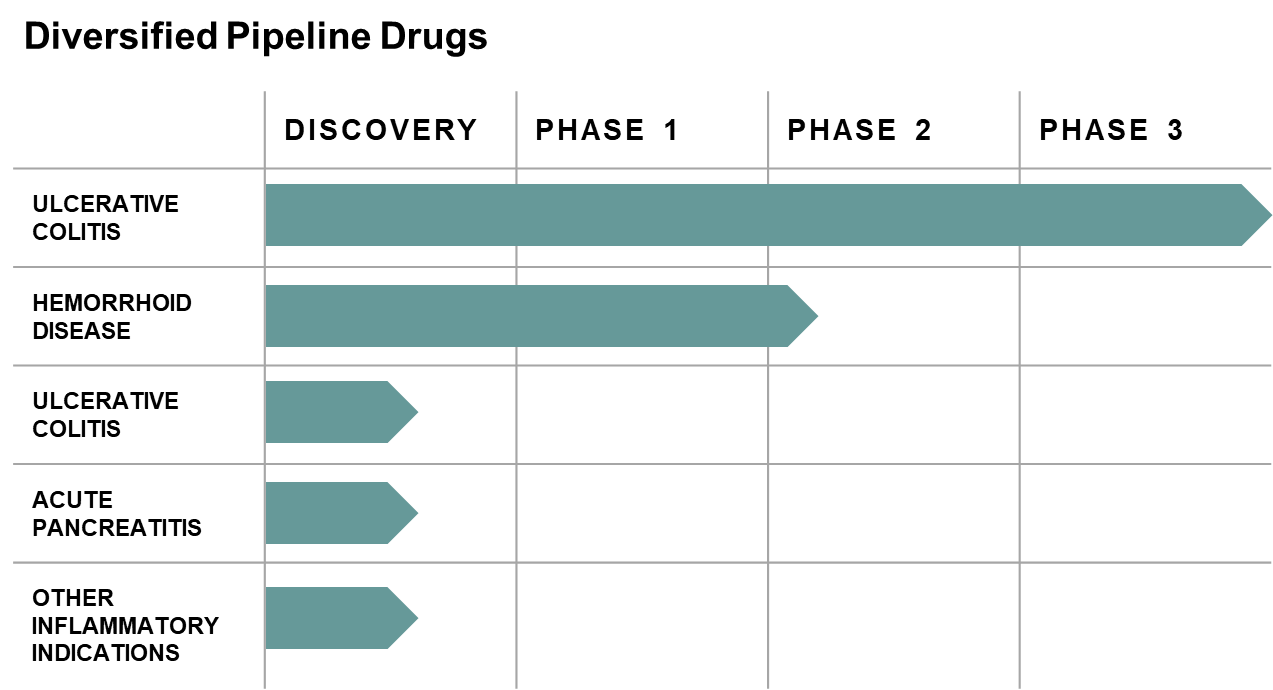
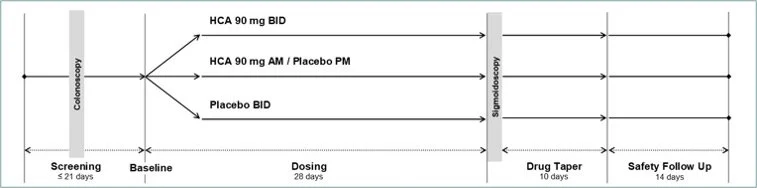
Phase 3 Clinical Trial in ulcerative colitis - Study Completed
Received Fast Track designation from the FDA, advancing the program into a pivotal Phase 3 clinical trial
Positive Results and Top Line Data
To read the announcement of study results, Click Here
Primary Endpoint:
Clinical remission measured using the Modified Mayo Score
Secondary Endpoints:
Reduction of stool frequency measured using Mayo Scoring sub-score of stool frequency
Rectal bleeding measured using Mayo Scoring sub-score of rectal bleeding equal to ‘0’